Nowadays, almost everyone is facing some kind of health issue, whether it’s a small allergy, a long-term illness, or something in between. Changing lifestyles and environmental factors are making people more dependent on medicines.
But have you ever thought about what happens before a medicine reaches you?
It’s the pharmaceutical industry that makes this possible. It plays a big role in global healthcare, but it’s also one of the most complex and highly regulated industries because of its many different processes.
But don’t worry, I am here to simplify it for you. Today in this case study, we’ll explore the key aspects of the pharmaceutical industry, from its working process to its supply chain and economic impact, with a special focus on India.
Types of Pharmaceutical Companies
The pharmaceutical industry is mainly about research, research, and more research. But this research needs a lot of money, time, and effort. Based on how much research a company does, we can divide them into three types: Let’s understand them one by one.
1. NCE Companies: The Discoverers
NCE (New Chemical Entity) companies are those that find new chemical substances that might be used as medicines in the future. These companies work in labs and study different molecules to see if they can treat diseases like cancer, diabetes, or infections.
But finding a new molecule is just the first step. Most of the time, these companies sell or give licenses for their discoveries to bigger pharmaceutical companies, which then turn them into actual medicines.
2. Innovator Companies: The Medicine Makers
Innovator companies take the new molecules found by NCE companies and develop them into real medicines. They spend years doing tests and clinical trials to make sure the medicines are safe and effective before they are sold in the market.
Since this process takes a lot of money, innovator companies get patents. A patent gives them the exclusive right to sell the medicine for a certain number of years. This helps them recover their investment and earn profit.
All innovator companies can be NCE companies, but not all NCE companies become innovators.
Some well-known innovator companies are Pfizer, Novartis, and Merck. They have introduced many important medicines.
3. Generic Companies: The Affordable Option
Once the patent of an innovator drug expires, generic companies can step in. They apply for approval (ANDA - Abbreviated New Drug Application) to use the same formula as the original medicine. Since they don’t spend money on research, their costs are much lower. This allows them to produce medicines at cheaper rates and in large quantities, making healthcare more affordable and accessible.
Most Indian pharmaceutical companies specialize in generic medicines, making healthcare more affordable worldwide. India’s pharma sector contributes 20% of the global supply of generic medicines, playing a key role in ensuring cost-effective treatment for people across the world.
Generic companies can be divided into three types:
Branded Generic Companies: Branded generic companies manufacture generic medicines but sell them under their brand names. These medicines are promoted through doctors, and the branding creates a sense of trust among patients. Since these companies spend on marketing, the price is slightly higher than unbranded generics. However, they are still cheaper than original patented drugs.
Some of the leading branded generic companies in India include Sun Pharma, Cipla, Dr Reddy’s, and Lupin.
Trade Generic Companies: Trade generic companies do not invest in marketing through doctors. Instead, they sell directly to medical stores, stockists, and distributors. These medicines are usually cheaper than branded generics.
Generic-Generic (Unbranded Generic) Companies: Genericgeneric companies produce medicines under their chemical names, without any branding. These medicines are primarily supplied to government hospitals, Jan Aushadhi Kendras, and public health programs. Since there is no branding or marketing cost, these are the most affordable medicines available.
Essential Terms for Understanding Pharma Companies
Now that we know the types of companies in this sector, let’s understand some key terminologies that will help in understanding how pharmaceutical companies operate and how their performance is measured.
Key Starting Material (KSM)
Every medicine starts with Key Starting Materials (KSMs), which are the basic chemicals needed to make the active part of a drug. They are the first ingredients needed to make the final product.
For the tablets like paracetamol, the KSM is Para-Aminophenol (PAP). PAP is produced using phenol and nitric acid, which undergo chemical reactions to form Para-Aminophenol.
For many years, India has imported KSMs from China because they were cheaper. However, now Indian companies like Laurus Labs, Aarti Industries, and Deepak Nitrite are making KSMs in India.
Intermediates
Once KSMs are made, they are converted into Intermediates, which are semi-processed chemicals. These intermediates are not medicines yet, but they are one step closer to becoming them.
In the case of Paracetamol, PAP is further refined and purified to make it suitable for converting into Active Pharmaceutical Ingredients (API).
Companies like Neuland, Blue Jet, AMI Organics, and SAF produce these intermediates and supply them to the next stage of the pharma process.
Active Pharmaceutical Ingredient (API)
The most important part of any medicine is the Active Pharmaceutical Ingredient (API). APIs are the active salts that treat your illness. almost 40% of the cost of medicine is of API. It is believed that the more APIs a company has, it is likely to see more growth.
The API market is divided into two segments:
Patented APIs: They are the active ingredients used in innovative or brand-name drugs, often protected by patents. These APIs are typically complex, requiring extensive research and clinical trials for development, which makes them more expensive. They are commonly found in medications for complex conditions like certain cancers or multiple sclerosis.
Generic APIs: They are used in generic drugs that are produced after the original patents expire. These APIs contain the same active substances as the original branded drugs but focus on replicating existing drugs without extensive research, leading to shorter development times and more affordable costs. Common medications like generic versions of ibuprofen or metformin are examples of drugs containing generic APIs.
Contract Research, Development, and Manufacturing Organizations (CRDMOs)
Many big pharmaceutical companies do not make their medicines. Instead, they give the work to other companies that specialize in making medicines. These companies are called CRDMOs/CDMOs/CROs/CMOs.
For example, a company like Glenmark or Hikal Limited might produce Paracetamol tablets for another big company, which will later sell them under its brand name.
The CRDMO industry can be futher bifurcated into various types:
CRDMOs: Contract Research, Development, and Manufacturing Organizations (CRDMOs) provide end-to-end services for making medicines. This means they help in drug discovery, development, and manufacturing, all in one place. They offer cost-effective and customized solutions, helping pharmaceutical companies develop medicines faster, reduce costs, and get expert help that they may not have within their own company.
CROs: Contract Research Organizations (CROs) focus only on scientific research, including drug discovery, preclinical testing, and clinical trials.
CMOs: Contract Manufacturing Organizations (CMOs) companies only handle medicine production. They do not engage in research but specialize in manufacturing medicines at different scales, ensuring high-quality and efficient production for pharmaceutical companies.
CDMOs: They help with developing drug formulations and large-scale manufacturing. They specialize in making medicines ready for sale and help pharma companies increase production when demand is high.
Most companies want to offer all services and become full CRDMOs, but in reality, they focus more on either research (services) or manufacturing (products).
Value Chain of Pharma Companies
Now that you know the key jargon used in the pharma sector, it will be easier for you to understand its nuances. Let’s now look at the value chain of pharma companies and how each stage plays a role in bringing medicines to people.
Stage 1: Research & Discovery
In this stage, scientists and pharmaceutical companies work to find a new chemical that can help treat a disease. NCE companies or Innovator companies play a crucial role in this process, as they specialize in discovering and developing new medicines. These companies invest heavily in research to create better treatments for diseases.
If an NCE company or a research center discovers a new molecule, they usually sell the process to large Innovator companies or make a royalty deal with them. This is because NCE companies are smaller and cannot manufacture medicines on a large scale like Innovator companies.
Since research is expensive and time-consuming, companies often collaborate with CRDMOs and CROs. They provide research support, conduct early-stage experiments, and assist in preclinical studies, helping to advance potential drug candidates more efficiently.
Stage 2: Development
Once a new chemical is found, it needs to be developed into a medicine. The first step is preclinical testing, where scientists conduct lab experiments and animal testing to check if the drug is safe. If the results are positive, the medicine moves to clinical trials, which are done in four phases.
Phase I: A small group of healthy people take the medicine to check its safety.
Phase II: A larger group of patients take the medicine to see if it works.
Phase III: Thousands of patients take the medicine, and doctors check its effectiveness and safety.
Phase IV: Even after approval, the medicine is monitored for any long-term side effects.
If the medicine successfully passes these trials, it is submitted to the US FDA for regulatory approval. This process is expensive and can take even 10 to 15 years.
At this stage, innovator companies take the lead. These companies focus on conducting clinical trials, obtaining regulatory approvals, and ensuring that their drug meets safety and efficacy standards. Since development is resource-intensive, many innovator companies partner with CRDMOs and CROs.
Stage 3: Manufacturing
Once approved, the medicine is produced in large quantities. Manufacturing involves three key steps:
Key Starting Material (KSM)
Intermediates
Active Pharmaceutical Ingredient (API)
Many pharmaceutical companies outsource manufacturing to CDMOs to reduce costs, maintain quality, and focus on innovation. Some generic pharmaceutical companies also rely on CDMOs and CRDMOs for bulk production of widely used medicines.
Stage 4: Commercialization
The final stage is when the medicine is ready to be sold in the market. Pharmaceutical companies promote their medicines to doctors, who then prescribe them to patients.
At this stage, different types of generic companies (as discussed earlier) play an important role. They market and distribute medicines on different levels and ensure they reach various places.
To make this process easier and smoother, companies also work with CRDMOs, CDMOs, or CMOs, who help with production and supply.
This ensures that important medicines are available to everyone at affordable prices.

Indian CRDMO Landscape
The Indian CRDMO industry is growing rapidly because more global pharmaceutical companies are choosing India for their research and manufacturing work. This is mainly because medicine costs are rising, and global supply chains are changing due to political and economic reasons.
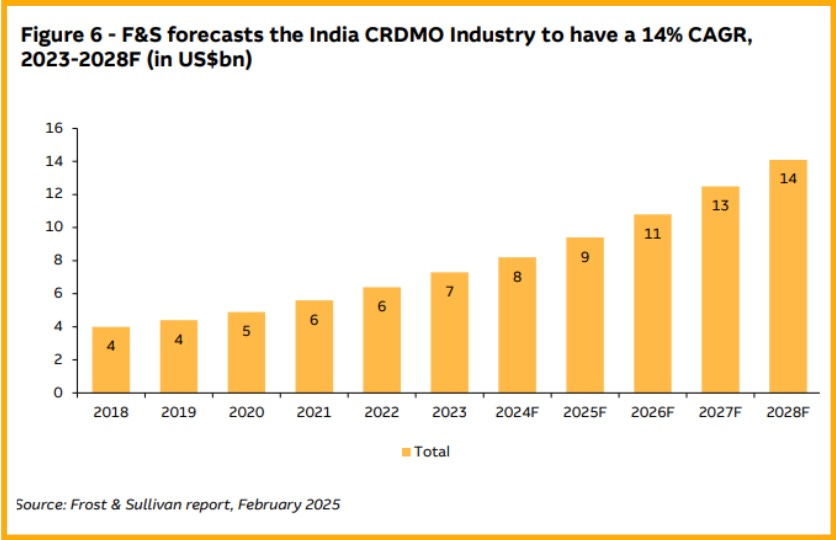
At present, the Indian CRDMO market is worth 7 billion US dollars. Experts say this number will double to 14 billion US dollars by 2028, growing at 14% per year.
Also, new government rules in other countries are creating more opportunities for the Indian CRDMO industry. One such rule is the US Biosecure Act, which stops US government agencies from using biotechnology from companies that may be a security risk.
Earlier, Chinese CDMOs were the most preferred because they were fast and experienced. But now, due to this new rule, many pharmaceutical companies are shifting their projects to other countries, which is slowing down China's growth in this sector.
This situation can benefit India because many companies are searching for reliable and affordable options. If this trend continues, India’s CRDMO market could grow even faster, reaching 22 billion US dollars by 2030.
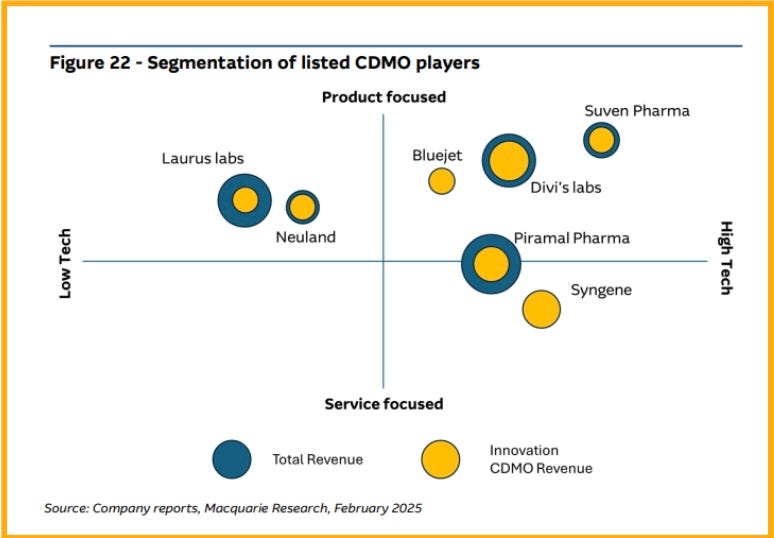
Among Indian CRDMO companies:
Syngene earns most of its money from research services.
Divi’s Labs and Suven Pharma focus more on manufacturing medicines.
Neuland, Piramal, and Laurus provide both research and manufacturing, but their focus is balanced between the two.
Indian CDMO companies can also be grouped based on two factors:
Technology intensity (How advanced their technology is).
Focus on products or services (Whether they earn more from making medicines or from research work).
Suven Pharmaceuticals and Divi’s Laboratories mainly focus on manufacturing medicines and work on advanced drug technologies like Antibody-Drug Conjugates (ADC) and Glucagon-Like Peptide-1 (GLP-1).
Syngene International gets about 60% of its revenue from research services.
Experts believe that CDMOs that focus on high-tech product focused will grow faster and make higher profits in the future.
That’s all from our end for this month’s case study!
Hopefully, this write-up has built a basic foundation in India’s pharmaceutical sector and its key players. But why stop here? Join our 100-day LinkedIn challenge!
Pick an industry, research it for 100 days, and share your insights on LinkedIn. This hands-on approach will help you dive deeper and stand out.
If you’ve never posted on LinkedIn or aren’t sure how to keep going, don’t worry! Just remember Zig Ziglar’s words:
"You don’t have to be great to start, but you have to start to be great."
Watch the video below for details!
That said, I’d love to hear your thoughts on the pharmaceutical sector or any topics you’d like us to cover in the future. Feel free to drop your comments below!
And don’t forget to like, share, and restack
Song of the Week:
This is Parth Verma,
Signing off.





Hi Sir,
You have not covered anything about Formulations here. Can you help me understand about it in simple terms?
Really enjoyed this! Had been researching some pharma stocks and this came just in time.
Suggest you to cover all the various sectors aswell. Would love to learn about Power sector. Thanks